Polymyxin B sulfate inducing time-dependent antagonism of the mixtures of pesticide, ionic liquids, and antibiotics to Vibrio qinghaiensis sp.-Q67†
Abstract
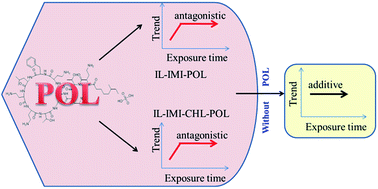
In the real environment, organisms are simultaneously exposed to different types of chemicals. Different types of chemicals usually have different time-dependent toxicity characteristics, however, people know little about the time-dependent toxicities of mixtures. Hence, the time-dependent toxicity of one pesticide, two ionic liquids (ILs), two antibiotics 20 ternary mixture rays and ten quaternary mixture rays to Vibrio qinghaiensis sp.-Q67 (V. qinghaiensis) were determined by the time-dependent microplate toxicity analysis. The results showed that the toxicity of pesticide (imidacloprid, IMI) to V. qinghaiensis does not change with time, the toxicities of two ILs (1-hexyl-3-methyl-imidazolium bromide, [hmim]Br, and 1-hexyl-3-methylimidazolium chloride, [hmim]Cl) decrease with time, and those of two antibiotics (chloramphenicol, CHL, and polymyxin B sulfate, POL) increase with time. The mixture toxicity almost doesn't change with time. Taking the concentration addition model as an additive reference, the combined toxicities of chemicals are assessed. It is shown that the mixture toxicities of most rays in two ternary mixture systems, [hmim]Br–IMI–CHL and [hmim]Cl–IMI–CHL, are additive at any time, while those in [hmim]Br–IMI–POL and [hmim]Cl–IMI–POL systems are antagonistic at some time, which implies that the POL may be a key component inducing the antagonism. To further validate the antagonism induced by POL, the POL is respectively merged into two ternary [hmim]Br–IMI–CHL and [hmim]Cl–IMI–CHL systems having no antagonism to form two quaternary mixture systems. It is found that many rays in the two new quaternary mixture systems have antagonistic action at some times, which validates that POL is indeed a key component. These findings indicate that the mixture toxicity of pesticide, ILs and antibiotics is complicated and prone to generate interaction, and doing risk assessment should take this type of mixtures into consideration.
- This article is part of the themed collection: Ionic Liquids: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...