Absolute ion hydration enthalpies and the role of volume within hydration thermodynamics†
Abstract
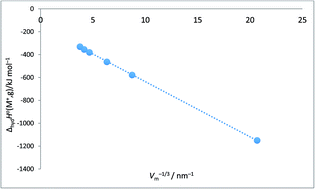
This paper reports that various thermodynamic properties in aqueous media for certain individual ions and for compounds are linear functions of the inverse cube root of the solid respective ionic and compound solid state volumes, Vm−1/3. This is similar to the situation which has been fully exploited in solid state thermodynamics and out of which volume-based thermodynamics, VBT, evolved. A short resume of these various VBT applications is provided for the general reader and an improved lattice potential energy equation emerges using the state of the art data presented in this paper.


 Please wait while we load your content...
Please wait while we load your content...