Functionalisation of ligands through click chemistry: long-lived NIR emission from organic Er(iii) complexes with a perfluorinated core and a hydrogen-containing shell†
Abstract
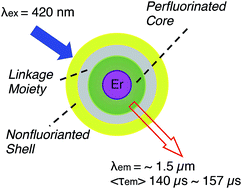
Erbium complexes with a fluorinated organic core shell linked to a hydrogen-containing shell, have been synthesized using the click reaction between erbium(III) bis(perfluoro-4-azidophenyl)phosphinate and a series of alkynes. The erbium 1.5 μm emission lifetimes in the hydrogen-containing erbium complexes exceed 140 μs, the longest ever reported in hydrogenated organic erbium systems. The visible sensitisation for erbium emission indicates a successful strategy that broadens the usage of non-fluorinated chromophores in organic erbium systems and allows more choices for ligand functionalization with exceptional efficiency for erbium emission.

 Please wait while we load your content...
Please wait while we load your content...