Manipulating energy storage characteristics of ultrathin boron carbide monolayer under varied scandium doping†
Abstract
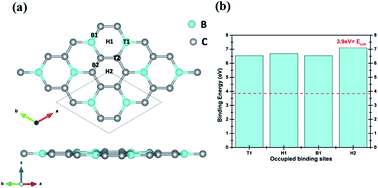
We report, for the first time we believe, a detailed investigation on hydrogen storage efficiency of scandium (Sc) decorated boron carbide (BC3) sheets using spin-polarized density functional theory (DFT). We analyzed the energetics of Sc adsorption and explored the most favorable adsorption sites of Sc on BC3 sheets with 3.12%, 6.25%, and 12.5% coverage effects. Our investigations revealed that Sc strongly binds on pristine BC3 sheet, with a minimum binding energy of ∼5 eV, which is robust enough to hinder Sc–Sc metal clustering. Sc, the lightest transition metal, adsorbs a large number of H2 molecules per atom, resulting in a reasonable storage capacity. With 12.5% Sc-coverage, functionalized BC3 sheets could attain a H2 storage capacity of 5.5 wt% with binding energies suitable for a practical H2 storage medium.


 Please wait while we load your content...
Please wait while we load your content...