Nanostructured ZnO thin films for self-cleaning applications
Abstract
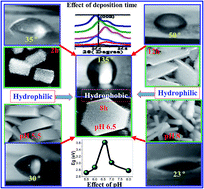
Controlling the properties of nanostructured zinc oxide (ZnO) is an interesting way to broaden its multifunctionality. ZnO nanostructured films were grown on glass substrates under different conditions by a simple two-step wet chemical method. A low-cost successive ionic layer adsorption and reaction (SILAR) method was used to grow ZnO seed layers at 80 °C. Then, different hierarchical based ZnO nanostructured thin films were deposited onto the ZnO seed layers by chemical bath deposition (CBD). The influence of deposition time (tD) and pH on the surface morphology, wettability behavior, structural and optical properties of the ZnO nanostructured films were systematically investigated. The structural, morphological, optical and wetting properties were studied by X-ray diffraction (XRD), field emission scanning microscopy (FE-SEM), UV-Vis spectrophotometer, and water contact angle (WCA) measurement, respectively. The surface morphology revealed a complex and orientated hierarchical based ZnO nanostructured films with diverse shapes from hexagonal nanorods to hexagonal nanoplates and even much more complex plates/rods and flower-like shapes by changing deposition time and pH of the precursor. XRD results confirm the synthesis of nanostructured ZnO of the hexagonal structure with a preferential orientation along the (002) lattice plane. The average crystallite size, D, is altered between 41.41 to 68.43 nm dependent on the morphology of the ZnO film and pH of the precursor. At pH 6.5, the films are hydrophilic for 10 h ≤ tD ≤ 6 h and hydrophobic for 6 h < tD < 10 h. The wetting properties of the films were enhanced by increasing or decreasing pH around 6.5. Morphology and thickness of the ZnO nanostructure could efficiently control the transmittance, absorbance, optical band gap, and the extinction coefficient of the films. The optical band gap is blue shifted from 2.45 to 3.62 eV@pH 6.5 as the deposition time increased from 2 to 8 h and blue shifted from 2.72 to 3.62 eV@8 h as the pH value increased from 5.5 to 6.5. The existence of stable hydrophobic zinc oxide nanostructured films at room temperature at a large-scale and with band gaps around 3.62 eV supports their use in self-cleaning and gas sensing applications.
- This article is part of the themed collection: RSC Advances: Most downloaded articles of 2017


 Please wait while we load your content...
Please wait while we load your content...