The effect of the nature of organic acids and the hydrodynamic conditions on the dissolution of Pb particles
Abstract
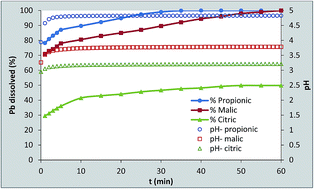
A series of experiments were conducted to examine the effects of three organic acids (propionic, malic and citric acids) on the rate of Pb dissolution in order to evaluate its potential environmental pollution risk. The effects of acid concentration, agitation device, temperature and hydrodynamic conditions were investigated. The results show that propionic acid which contained the lowest number of functional groups had the greatest effect on Pb dissolution. In fact, it dissolved 100% of Pb particles after 35 min at the concentration of 5 mM at 25 °C while 92.1 and 47.6% were dissolved by malic and citric acid, respectively, under the same conditions. It was also found that the effect of the nature of the acid interfered with that of particle size and the hydrodynamic conditions making the system complicated to interpret totally.

 Please wait while we load your content...
Please wait while we load your content...