Polymer-supported benzimidazolium based ionic liquid: an efficient and reusable Brønsted acid catalyst for Biginelli reaction†
Abstract
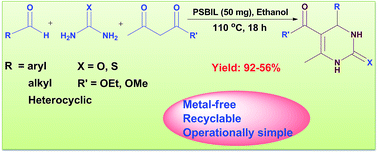
A polymer-supported benzimidazolium based ionic liquid (PSBIL) was synthesized by reaction of poly(vinylbenzyl chloride) and benzimidazole followed by ring opening of 1,4-butane sultone and acidification with sulphuric acid. The resulting Brønsted acid heterogeneous organocatalyst is very competent for the Biginelli reactions under mild conditions in high yields of 3,4-dihydropyrimidine-2(1H)-one/thiones (DHPMs). Since poly(vinylbenzyl chloride) makes the catalyst bed, the sulphonic acid catalyst will have high loading level of acidic groups compare to other heterogeneous acid catalysts. In spite of conventional heterogeneous ionic liquid catalysts, current PSBIL shows thermal stability up to 334 °C and recyclability up to 5 cycles. This PSBIL signifies a new class of heterogeneous catalyst which is particularly noticeable in green chemistry. The present protocol highlights the easy separation of products without further purification. Furthermore, the effectiveness of this protocol has demonstrated by synthesis of mitotic Kinesin EG5 inhibitor, “Monastrol” under optimized conditions.

 Please wait while we load your content...
Please wait while we load your content...