Materials for aqueous sodium-ion batteries: cation mobility in a zinc hexacyanoferrate electrode†
Abstract
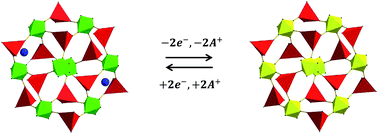
The coordination polymer Zn3Na2[FeII(CN)6]2 has an open porous framework that is stable in acidic and neutral aqueous solutions and appears to be an attractive solid for investigation as a material for sodium ion-based batteries. The easy ion exchange of Na+ by bigger cations (such as K+, Rb+ and Cs+) and the existence of the oxidized species Zn3[FeIII(CN)6]2 (as a stable phase) support the present study of the redox reaction and stability in aqueous media under electrochemical conditions for all the series, including the effect of the accompanying cation on the mobility of sodium in the framework. When a study was carried out in a solution of sodium ions, in the material structure K+ and Rb+ were progressively displaced by the sodium ions, which was ascribed to their higher mobility. However, when an experiment was conducted using a solution containing Cs+ or in an aqueous solution of NaNO3, from Zn3Cs2[FeII(CN)6]2, the mixed composition Zn3NaCs[FeII(CN)6]2 was formed. The results discussed here are supported by the electrochemical and spectroscopic measurements. Galvanostatic experiments on the Zn3Cs2[FeII(CN)6]2 system in a solution of sodium ions revealed a capacity retention of 75% during 40 charge/discharge cycles.

 Please wait while we load your content...
Please wait while we load your content...