Ultra-sensitive fluorescence determination of chromium(vi) in aqueous solution based on selectively etching of protein-stabled gold nanoclusters†
Abstract
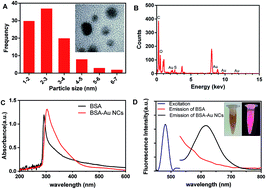
In this work, we have developed a simple, cost-effective and sensitive fluorescent method for the selective determination of chromium(VI) ions (Cr(VI)) in aqueous solution. In the presence of bromide ions (Br−), the strong oxidization of Cr(VI) enables it to selectively etch the bovine serum albumin-stabled gold nanoclusters (BSA-Au NCs) and formed AuBr2− complexes. Proportional fluorescence quenching of BSA-Au NCs induced by the leaching process was closely connected with concentrations of Cr(VI) and thus provided a new detecting strategy towards Cr(VI). Under optimal conditions, the amount-dependent fluorescent response was linearly correlated with the Cr(VI) concentrations ranging from 1.0 nM to 2.5 μM over 3 orders of magnitude, with a detection limit down to 0.6 nM. The as-developed strategy also performed satisfactory selectivity as well as excellent reproducibility. Moreover, this method had been successfully applied to monitor Cr(VI) with real river water samples which demonstrated the potential for field applications.

 Please wait while we load your content...
Please wait while we load your content...