Synthesis of 2-arylated thiadiazolopyrimidones by Suzuki–Miyaura cross-coupling: a new class of nucleotide pyrophosphatase (NPPs) inhibitors†
Abstract
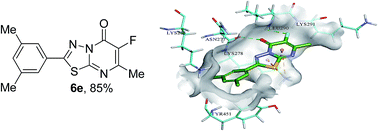
Over expression of nucleotide pyrophosphatase (NPPs) activity is associated with chondrocalcinosis, osteoarthritis, type 2 diabetes, neurodegenerative diseases, allergies and cancer metastasis. The potential of NPPs inhibitors as therapeutic agents, and the scarceness of their structure–activity relationship, encouraged us to develop new NPP inhibitors. Specifically, 2-bromo-7-methyl-5-oxo-5H-1,3,4-thiadiazolopyrimidine and its corresponding 6-fluoro derivatives were synthesized via a Suzuki–Miyaura reaction. The cross-coupling reaction with different arylboronic acids gave desired coupling products in good to excellent yields and showed wide functional group tolerance. Furthermore, all compounds were investigated for their potential to inhibit two families of ecto-nucleotidases, i.e. nucleoside triphosphate diphosphohydrolases (NTPDase) and NPPs. Interestingly, our compounds were identified as selective inhibitors of NPPs. Among derivatives 5a–5i, compound 5i (IC50 ± SEM = 0.39 ± 0.01 μM) was found to be the most potent inhibitor of h-NPP1 and compound 5h (IC50 ± SEM = 1.02 ± 0.05 μM) was found to be the most potent inhibitor of h-NPP3. Similarly, for fluorinated thiadiazolopyrimidones, derivative 6e (IC50 ± SEM = 0.31 ± 0.01 μM) exhibited the best inhibition of NPP1 and it was found that this compound exhibited ≈28 fold improvement in inhibitory potential as compared with the reference control i.e. Suramin (IC50 ± SEM = 8.67 ± 1.3 μM). Moreover, homology modelling and molecular docking studies of both inhibitors were carried out to suggest the putative binding mode of inhibitors with the respective enzyme i.e. h-NPP1 and h-NPP3.

 Please wait while we load your content...
Please wait while we load your content...