Isatin pentafluorophenylhydrazones: interesting conformational change during anion sensing†
Abstract
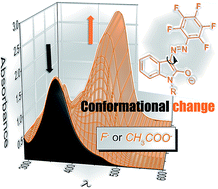
The anion sensing properties of two new easily synthesized isatin pentafluorophenylhydrazone reversible colorimetric chemosensors are studied herein. The F− or CH3COO− anion addition to isatin pentafluorophenylhydrazone solutions in aprotic organic solvents results in hydrazone NH group deprotonation of the initial Z-hydrazo form (acid–base keto/enolate reaction), with significant equilibrium shift to the more conjugated E-azo enolate side. Initial solutions thus turn orange. This fast equilibrium is followed by slow conformational change to the more stable E-azo enolate conformer with the configuration of the E-hydrazo isomer. Interestingly, the F− or CH3COO− detection in semi-aqueous media leads directly to slow formation of the second E-azo enolate conformer, without the observation of an initial fast equilibrium between Z-hydrazo and the corresponding first E-azo enolate conformer. Although the reaction time in semi-aqueous media thus increases to several minutes (tens of minutes), the advantage of isatin pentafluorophenylhydrazone chemosensors still remains their easy synthesis and their reversibility. The determined sensitivity towards F− and CH3COO− anions is among the highest published sensitivity to these anions in organic solvents and allows confident F− detection at the tolerated drinking-water fluoride level in semi-aqueous media.

 Please wait while we load your content...
Please wait while we load your content...