Understanding the roles of novel electron donors in Ziegler–Natta catalyzed propylene polymerization†
Abstract
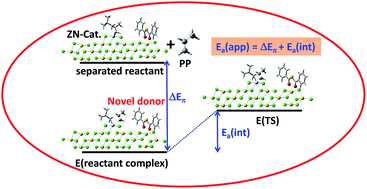
Roles of a novel dibenzoyl sulfide donor in Ziegler–Natta (ZN) catalyzed propylene polymerization were examined using DFT calculations. The adsorption mode for dibenzoyl sulfide and diisobutyl phthalate electron donors on the MgCl2(110) surface is the chelate coordination mode, similar to malonate electron donors. The dibenzoyl sulfide/diisobutyl phthalate donor provides regio- and stereo-selectivity to the ZN catalyst. Factors that control the isotacticity and activity of the ZN catalyst are steric repulsion and π-complex stabilization by electron donors. The steric repulsion signifies intrinsic activation energy and π-complex formation energy for π-complex stabilization. The two factors are combined in the apparent activation energy and dibenzoyl sulfide gives the lowest apparent activation energy. Therefore, dibenzoyl sulfide shows the best catalytic enhancement as compared to diisobutyl phthalate and di-n-butyl-2-cyclopentyl malonate, which is in good agreement with the results obtained from the experiments.

 Please wait while we load your content...
Please wait while we load your content...