Synthesis and characterization of two novel second-order nonlinear optical chromophores based on julolidine donors with excellent electro-optic activity
Abstract
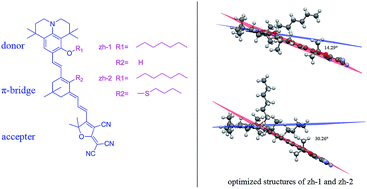
Julolidine and its derivatives are excellent electron-rich structures thus two novel second order nonlinear optical (NLO) chromophores based on julolidine donors and tricyanofuran (TCF) acceptors linked via isophorone bridge (zh-1) and modified isophorone bridge (zh-2) have been synthesized. Density functional theory (DFT) was used to optimize the geometrical structures, calculate the HOMO–LUMO energy gaps and first-order hyperpolarizability (β) of these chromophores. Besides, to determine the redox properties of these chromophores, cyclic voltammetry (CV) experiments were performed. The optimized structures suggest that chromophore zh-1 possesses better planarity which increases the ability of electron delocalization. These chromophores showed good thermal stability with their decomposition temperatures all above 210 °C. The electro-optic coefficients (r33) of poled films containing 25 wt% of these chromophores doped in amorphous polycarbonate (APC) afforded values of 170 and 101 pm V−1 at 1310 nm for chromophores zh-1 and zh-2, respectively. These properties show the potential use of the new chromophores as advanced material devices.

 Please wait while we load your content...
Please wait while we load your content...