Identification of a novel selective inhibitor of mutant isocitrate dehydrogenase 1 at allosteric site by docking-based virtual screening†
Abstract
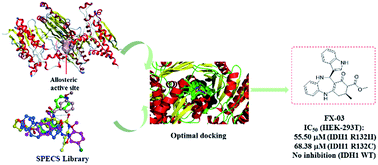
Isocitrate dehydrogenase 1 (IDH1), catalyzing oxidative decarboxylation of isocitrate to provide energy for aerobic organisms, is an essential enzyme in the tricarboxylic acid cycle. However, mutant IDH1 (mIDH1) produces oncometabolite D-2-hydroxyglutarate (D2HG) and has recently been confirmed in several types of cancers, particularly in glioma and acute myeloid leukemia. Herein a docking-based virtual screening (VS) of SPECS library was conducted for the allosteric site of mIDH1. The cellular evaluation of the hit compounds led to the identification of FX-03 as a novel selective mIDH1 inhibitor at allosteric site with IC50 values of 55.50 μM and 68.38 μM in HEK-293T cells transfected with IDH1 R132H and IDH1 R132C, respectively. Importantly, FX-03 owned significant selectivity with no inhibition in HEK-293T cells transfected with IDH1 WT. These findings indicate that VS of mIDH1's allosteric site represents a useful strategy for discovery of selective mIDH1 inhibitors and FX-03 deserves further optimization as a lead compound in future study.

 Please wait while we load your content...
Please wait while we load your content...