Synthesis, optical properties, and acid–base indicating performance of novel ketene hydroxybenzylidene nopinone derivatives†
Abstract
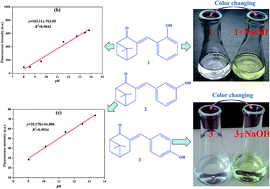
Three ketene nopinone derivatives (1–3) were successfully synthesized from β-pinene derivative nopinone and characterized by Fourier transform infrared spectroscope (FT-IR), nuclear magnetic resonance (NMR), mass spectrometry, and X-ray single crystal diffraction. Then, their optical properties were investigated by ultraviolet-visible and fluorescence spectroscopy. The ethanol solutions of compound 1 or 3 could change color from colorless to saffron yellow and Kelly, respectively, after adding NaOH solution. Moreover, the addition of potassium tert-butoxide could lead to a change in the UV-vis absorption and fluorescence spectra and enhance fluorescence, with compounds 1 and 3 being greatly affected by alkali. A good linear relationship between fluorescence intensity and pH values (9.01–13.38) was obtained for compound 2 (for detecting further pH value, y = 10.178x − 61.806, R2 = 0.9934). Compounds 1 and 3 were ideal acid–base indicators because of their high sensitivity, which was better than that of phenolphthalein. Therefore, these new nopinone derivatives may be candidates for future acid–base indicators.

 Please wait while we load your content...
Please wait while we load your content...