Copper-catalyzed one-pot oxidative amidation of alcohol to amide via C–H activation†
Abstract
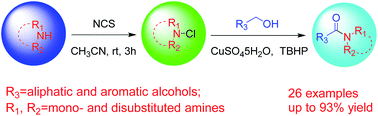
A one-pot oxidative amidation of both aliphatic and aromatic alcohols with N-chloramines, prepared in situ from many types of primary and secondary amines, was developed. This cross-coupling reaction integrates alcohol oxidation and amide bond formation, which are usually accomplished separately, into a single operation. And it was green, simple and convenient, which has a wide substrate scope and makes use of cheap, abundant, and easily available reagents. The practical value of this method is highlighted through the synthesis of a high-profile pharmaceutical agent, acetylprocainamide.


 Please wait while we load your content...
Please wait while we load your content...