Effect of NOx on product yields and Arrhenius parameters of gas-phase oxidation of β-ocimene initiated by OH˙ radicals†
Abstract
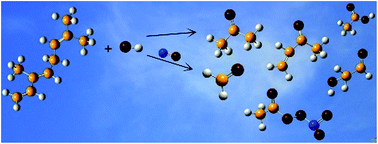
Rate coefficients for the gas-phase reaction of OH˙ radicals with β-ocimene were measured using the relative rate method over the temperature range 288–311 K at 760 Torr total pressure of nitrogen. The experiments were performed in a large volume environmental chamber using long-path FTIR spectroscopy to monitor the reactants. A room temperature rate coefficient of k(β-ocimene+OH˙)= (2.36 ± 0.54) × 10−10 cm3 molecule−1 s−1 was obtained for the title reaction. The temperature dependent rate coefficients are best fit by the Arrhenius expression k= (4.02 ± 0.71) × 10−14 exp(2567 ± 211)/T. In addition, product studies have been performed at (298 ± 2) K and 760 of Torr of synthetic air in the absence and presence of NOx. The following molar products were determined: formaldehyde (16.5 ± 0.9)% and (24.3 ± 1.5)%, acetone (45.6 ± 2.1)% and (58.3 ± 3.4)%, methyl vinyl ketone (18.5 ± 0.8)% and <5% and glycolaldehyde (7.6 ± 0.6)% and <5% in the absence and presence of NOx, respectively. Acetic acid (<5%) was only found in the reaction performed in the absence of NOx. With NOx peroxy acetyl nitrate was formed with a yield <5%. Reaction mechanisms accounting for the formation of the products are proposed and atmospheric implications are discussed.

 Please wait while we load your content...
Please wait while we load your content...