Heterogeneous photocatalytic ozonation of ciprofloxacin using synthesized titanium dioxide nanoparticles on a montmorillonite support: parametric studies, mechanistic analysis and intermediates identification†
Abstract
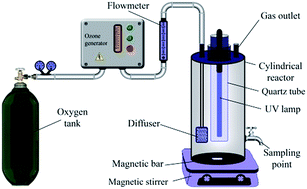
A titanium dioxide/montmorillonite (TiO2/MMT) nanocomposite was prepared as a photocatalyst by a hydrothermal method. The physicochemical properties of the prepared sample were comprehensively characterized using scanning electron microscopy (SEM), electron dispersive X-ray spectroscopy (EDX), high resolution transmission electron microscopy (HRTEM), Fourier transform infrared spectroscopy (FTIR), X-ray fluorescence (XRF), N2 adsorption–desorption, ultraviolet-visible diffuse reflectance spectroscopy (UV-vis DRS), and the pH of the zero point of charge (pHzpc) analysis. The photocatalytic ozonation of ciprofloxacin (CIP) was studied in the presence of the TiO2/MMT nanocomposite under different experimental conditions. Comparison of the main processes such as photocatalysis, ozonation and photocatalytic ozonation revealed that photocatalytic ozonation resulted in the highest degradation efficiency (90.00% at 30 min) of the pollutant under the optimum conditions ([CIP]0 = 20 mg L−1, [catalyst]0 = 0.04 g L−1, ozone gas flow rate = 2 L h−1 and pH = 5). This increase was due to a synergistic effect between photocatalysis and ozonation triggered by TiO2/MMT. The mechanism of the photocatalytic ozonation process was investigated in the presence of various organic and inorganic reactive oxygen species (ROS) scavengers. Accordingly, among the radical scavengers, the iodide ions and benzoquinone showed the highest inhibitory effect on the degradation efficiency of CIP. The photocatalytic ozonation mechanism of the TiO2/MMT nanocomposite for the degradation of CIP was thoroughly investigated. The performance of the photocatalytic ozonation process in a real water matrix was evaluated using well and ground water samples. In addition, the reusability of the TiO2/MMT nanocomposite in the photocatalytic ozonation process was examined. The result showed that the degradation efficiency of CIP declines by only about 7% after four consecutive runs. The main degradation intermediates of CIP produced in the photocatalytic ozonation process were identified by gas chromatography coupled to mass spectrometry (GC-MS) analysis.

 Please wait while we load your content...
Please wait while we load your content...