Catalytic etherification of glycerol to tert-butyl glycerol ethers using tert-butanol over sulfonic acid functionalized mesoporous polymer†
Abstract
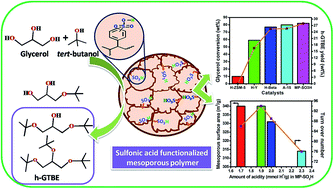
Mesoporous polymers (MP) were synthesized by free radical polymerization of divinylbenzene by a solvothermal method followed by sulfonic acid functionalization by a post synthetic modification with conc. H2SO4. MP-SO3H was characterized by various physicochemical techniques such as FT-IR, nitrogen sorption, CHNS analysis, acid–base titration and TGA. MP-SO3H was demonstrated to be a highly active heterogeneous acid catalyst, providing a high yielding route for the synthesis of h-GTBE through the etherification of bio-renewable glycerol with tert-butanol. MP-SO3H containing optimum acidity and a large mesoporous surface area gave 44% h-GTBE selectivity at 86% glycerol conversion under optimized reaction conditions. The catalytic performance of MP-SO3H was found to be superior over other conventional porous solid acid catalysts.

 Please wait while we load your content...
Please wait while we load your content...