The green preparation of poly N-vinylpyrrole nanoparticles†
Abstract
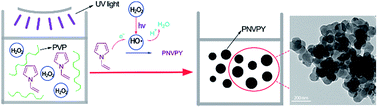
Well-dispersed poly N-vinylpyrrole (PNVPY) nanoparticles have been synthesized by UV-catalytic chemical polymerization with hydrogen peroxide (H2O2) as the oxidant and polyvinyl pyrrolidone (PVP) as the stabilizer. The chemical structure of PNVPY was characterized by FTIR and 1H-NMR. The morphology and size of PNVPY nanoparticles were observed and tested. It was found that the PNVPY particles were spherical and the particle size ranged from 18 to 106 nm with an average value of 25.98 nm. The effect of experimental conditions on the formation process of polymer nanoparticles was investigated. It was found that the polymerization rate of N-vinylpyrrole (NVPY) and the average size of PNVPY increased with the increase of UV irradiation intensity from 0 to 30 W and H2SO4 concentration from 2 to 22 g L−1. Meanwhile, with the increase of PVP concentration from 5 to 20 g L−1, the polymerization rate increased while the average size decreased. In addition, it was interesting that PNVPY nanoparticles had good compatibility with polyethylene glycol dimethacrylate due to the introduction of the vinyl group.

 Please wait while we load your content...
Please wait while we load your content...