Synthesis, structure and photochromic properties of hybrid molecules based on fullerene C60 and spiropyrans†
Abstract
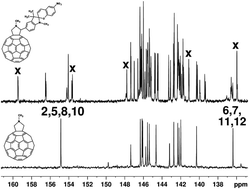
The 1,3-dipolar cycloaddition of azomethine ylides to fullerene C60 was utilized to perform the synthesis of spiropyran-containing photochromic pyrrolidinofullerenes. It was found that photochromism is observed only for the hybrid compound containing an NO2 group in the pyran moiety, whereas the pyrrolidinofullerenes with Cl or F atoms in the spiropyran moiety do not possess photochromism. The photochromic fullerene hybrid is characterized by lower light sensitivity and photodegradation parameters compared to the initial spiropyrans, which can be attributed to reabsorption of activating radiation.


 Please wait while we load your content...
Please wait while we load your content...