Photocatalytic degradation of two different types of dyes by synthesized La/Bi2WO6†
Abstract
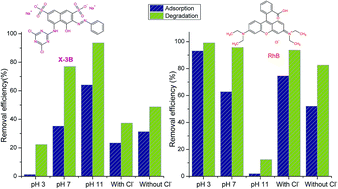
A lanthanum doped Bi2WO6 composite (La/Bi2WO6) was prepared using a simple hydrothermal method. The synthesized samples were well characterized using XRD, SEM, TEM, UV-vis DRS, XPS, and BET analysis methods. The photocatalytic activities of La/Bi2WO6 on reactive brilliant red X-3B (X-3B) and rhodamine B (RhB), under simulated solar irradiation, were systematically investigated. As a result, the doped Bi2WO6 samples possessed a higher specific surface area than pure Bi2WO6, but still retained the crystalline phases, morphology and visible light absorption properties of pure Bi2WO6. The initial concentrations of dyes, the dosage of catalyst and H2O2 all displayed the same effects on the degradation of the two dyes. However, pH showed different effects on X-3B and RhB because X-3B is an anionic dye while RhB is a cationic dye. Moreover, among all the inorganic ions studied (Na+, K+, Ca2+, Mg2+, NO3−, HCO3−, Cl−, SO42−), the addition of Cl− significantly affected the adsorption and degradation of the two dyes; Cl− inhibited the total removal efficiency of X-3B, but enhanced the removal efficiency of RhB. The effects of inorganic cations were attributed to the effect of Cl− because the four metal ions were used as their chloride salts.

 Please wait while we load your content...
Please wait while we load your content...