Shikonin derivatives as inhibitors of tyrosyl-tRNA synthetase: design, synthesis and biological evaluation
Abstract
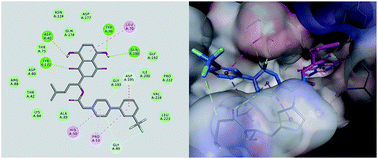
The development of antibiotics is never a simple stepwise linear process. After numerous setbacks in high-throughput screening and de novo drug design, researchers in this area are turning again to natural products. Shikonin, which is isolated from Lithospermum erythrorhizon, has multifarious pharmacological properties including antibacterial activity. In this study, we utilized shikonin as a template to grow a scaffold and acquire new molecules as tyrosyl-tRNA synthetase inhibitors. Docking simulation combined with a primary screen in vitro was performed to filter the modifications for the lead compound, which was then substituted, synthesized and evaluated for its inhibitory activity against TyrRS and several strains of both Gram-positive and Gram-negative bacteria. Out of the obtained compounds, PMM-154 exhibited high potency with an IC50 of 0.8 ± 0.3 μM against TyrRS. In addition, improved potency against S. aureus ATCC 25923 with an MIC of 1.1 μg mL−1 was observed. In subsequent tests, the results showed that PMM-154 could cause the apoptosis of S. aureus ATCC 25923 at a safe concentration. In general, the biological results suggested that this shikonin derivative outperformed shikonin and may be further developed as a promising TyrRS inhibitor.


 Please wait while we load your content...
Please wait while we load your content...