20-Nor-isopimarane cycloethers from the deep-sea sediment-derived fungus Aspergillus wentii SD-310†
Abstract
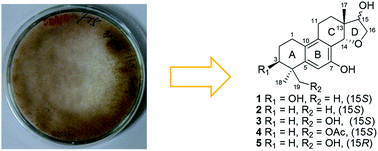
Asperethers A–E (1–5), five new 20-nor-isopimarane diterpenoids having a 14,16-cyclic ether unit and possessing a unique 6/6/6/5 tetracyclic skeleton, which had not so far been described for this class of diterpenoids, were identified from the culture extract of Aspergillus wentii SD-310, a fungus obtained from a deep-sea sediment sample. The structures of these compounds were established on the basis of detailed interpretation of NMR and mass spectroscopic data, and their relative and absolute configurations were confirmed by NOESY data and X-ray crystallographic analysis, as well as by TDDFT calculations of their ECD spectra. Compounds 1–5 showed cytotoxic activities against several tumor cell lines.

 Please wait while we load your content...
Please wait while we load your content...