Elemental sulfur mediated synthesis of benzoxazoles, benzothiazoles and quinoxalines via decarboxylative coupling of 2-hydroxy/mercapto/amino-anilines with cinnamic acids†
Abstract
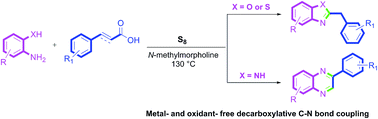
An easy and practical method has been developed for the synthesis of 2-benzylbenzoxazoles and 2-benzylbenzothiazoles using sulfur mediated decarboxylative coupling of cinnamic acids with 2-hydroxyanilines and 2-mercaptoanilines respectively under metal- and solvent-free conditions. However, the reaction of 2-aminoanilines with cinnamic acids leads to the formation of 2-arylquinoxalines under the same set of reaction conditions. The transformation is versatile and compatible with a number of functional groups.

 Please wait while we load your content...
Please wait while we load your content...