Influence of structure–property relationships of two structural isomers of thiophene-flanked diazaisoindigo on carrier-transport properties†
Abstract
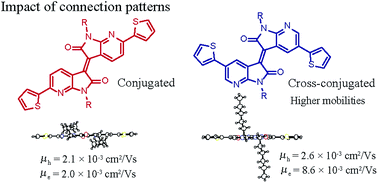
Based on the electron-accepting building block of 7,7′-diazaisosindigo (DAII), two structural isomers of thiophene-flanked diazaisoindigo, 6,6′-substituted 6,6′-T-DAII for full conjugation and 5,5′-substituted 5,5′-T-DAII for cross-conjugation, have been designed and synthesized to study the influence of the connecting positions of the flanking thiophene on the optoelectronic properties, crystal structures, and solid-state microstructures, being well associated with the carrier-transport properties. The results indicate that the 5,5′-substitution on the DAII core of 5,5-T-DAII stabilizes both the HOMO and LUMO levels and reduces the oscillation strength of the low-energy absorption band as compared to the 6,6′-substitution. In the crystals, 6,6′-T-DAII, 5,5′-T-DAII, and 6,6′-dibromo-DAII adopt a flat platform with co-facial slipped π–π stacking. The organic field-effect transistors based on 6,6′-T-DAII and 5,5′-T-DAII on a tetratetracontane (TTC) modified substrate exhibit an n-dominant ambipolar performance with the hole and electron mobilities of 10−3 cm2 V−1 s−1. Of particular note is that 5,5′-T-DAII show obviously higher carrier-mobilities compared to 6,6′-T-DAII, suggesting that the cross-conjugation of the 5,5′-connection on the DAII core could be designed to improve the carrier-transport properties for FET applications.


 Please wait while we load your content...
Please wait while we load your content...