Reduction of Mn3+ to Mn2+ and near infrared plasmonics from Mn–Sn codoped In2O3 nanocrystals†
Abstract
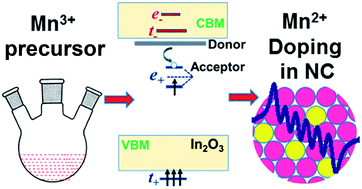
Colloidal Sn doped In2O3 nanocrystals have gained considerable attention for exhibiting surface plasmon resonance (SPR) which can be easily tuned in the near to mid infrared region by controlling the dopant concentration. Codoping these NCs with magnetic ions such as Fe3+, Mn2+/3+ can help develop interactions between delocalized electrons and localized magnetic spins which are required for spin-based applications. We prepared colloidal Mn–Sn codoped In2O3 nanocrystals with a diameter of ∼6–7 nm, starting from a Mn3+ precursor for Mn doping. Detailed characterization including Q-band electron paramagnetic resonance spectroscopy show that Mn exists in the 2+ oxidation state in both Mn-doped and Mn–Sn codoped In2O3 nanocrystals, in spite of using the Mn3+ precursor. This aliovalent Mn2+ doping along with charge neutralization through oxygen vacancies are energetically more favorable when compared to isovalent Mn3+ substitution in the In2O3 lattice. The SPR band decreased both in intensity and energy with increasing Mn content in the Mn–Sn codoped In2O3 nanocrystals. This is because Mn2+-doping introduces a hole in the lattice promoting electron–hole recombination reducing the free electron density. Also, Mn2+ dopants scatter the electrons thereby broadening the SPR band.

 Please wait while we load your content...
Please wait while we load your content...