Improvement on the high-rate performance of Mn-doped Na3V2(PO4)3/C as a cathode material for sodium ion batteries†
Abstract
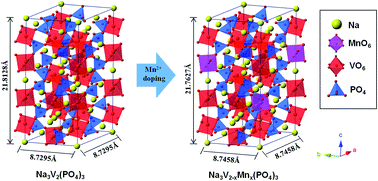
Mn2+ doped Na3V2−xMnx(PO4)3/C (x = 0, 0.015, 0.025 and 0.035) samples were synthesized by a facile sol–gel method and doping effects on the crystal structure and electrical conductivity were investigated by Rietveld refinement of XRD and a RTS-4 linear four-point probe. The results show that moderate doping of Mn2+ does not alter the structure of Na3V2(PO4)3, and Mn2+ successfully substituted partial V3+ sites. Due to the larger ionic radius of Mn2+ (0.91 Å) as compared to V3+ (0.64 Å), the lattice volume of Mn2+ doped Na3V2−xMnx(PO4)3/C noticeably increased, which could significantly accelerate Na+ transport in the material. Moreover, moderate Mn doping is in favour of increasing the electronic conductivity of Na3V2−xMnx(PO4)3/C samples. As a result, the Mn2+ doped Na3V2−xMnx(PO4)3/C samples show obvious improvements on the electrochemical performance in terms of the high-rate performance and cycling stability, particularly for the Na3V1.875Mn0.025(PO4)3/C sample. As an example, when the discharging rate is 15C, it can deliver an initial discharge capacity of 86.7 mA h g−1, and after 100 cycles, 79.4 mA h g−1 can still be achieved.

 Please wait while we load your content...
Please wait while we load your content...