pH dependent size control, formation mechanism and antimicrobial functionality of bio-inspired AgNPs†
Abstract
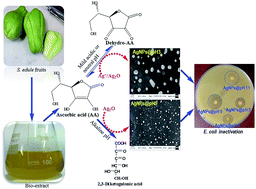
Sechium edule is rich in ascorbic acid which was extracted from aqueous media for the synthesis of AgNPs. This work reports the effects of pH on the kinetics and mechanisms of AgNPs formation using this extract leading to different particle sizes, morphology, spectral response, and antimicrobial functionality. Thermodynamically facile Ag2O reduction at a higher pH (≥9) resulted in spherical particles of smaller sizes; however, the particles were laden with a trace of Ag2O at pH 12.5. A broad and bimodal distribution of AgNPs of different shapes and sizes were originated at a lower pH (3 ≤ pH ≤ 5) from Ag+ and Ag2O reductions where ascorbic acid mostly exists as dehydro-ascorbic acid. A single surface plasmon resonance peak at 425 nm exhibited a blue shift with the decrease in AgNPs size and increase in sphericity in the abundance of OH− ions. The silver–ascorbate layer induced particles destabilization along with the formation of ascorbate free radical (g-factor: 2.0052 to 2.0056) which was also responsible for the inhibition of B. subtilis and E. coli.


 Please wait while we load your content...
Please wait while we load your content...