Magnetic nanoparticle (MNP)-supported 9-amino(9-deoxy)epi-quinidine organocatalyst for the asymmetric α-amination of aldehydes†
Abstract
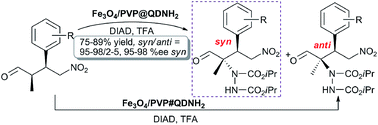
Fe3O4 MNP-supported 9-amino(9-deoxy)epi-quinidine (Fe3O4/PVP@QDNH2) with core–shell morphology and a high loading capacity of QDNH2 (0.60 mmol g−1) was prepared by an available one-pot method through the first homogeneous radical-mediated thiol–ene click reaction of QDNH2 with 3-mercaptotrimethoxysilane (MPTMS) and then hydrolysis of –Si(OCH3)3 on the surface of Fe3O4/PVP MNPs, and exhibited the good isolated yields (75–89%), excellent diastereoselectivities (syn/anti = 95–98/2–5) and remarkable enantioselectivities (95–98% ee syn) in the α-amination of (2R,3S)-2-methyl-3-nitro-4-phenylbutyraldehyde and its derivatives with di-iso-propyl azodicarboxylate (DIAD). Fortunately, the good diastereoselectivity (syn/anti = 88/12) and excellent enantioselectivity (95% ee syn) in the fifth run could be achieved in 75% yield.

 Please wait while we load your content...
Please wait while we load your content...