Organoaluminum(iii) complexes of the bis-N,N′-(2,6-diisopropylphenyl)imidazolin-2-imine ligand†
Abstract
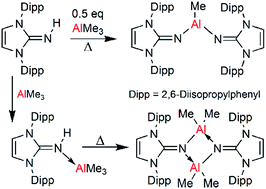
The reaction of trimethylaluminum with the bis-N,N′-(2,6-diisopropylphenyl)imidazolin-2-imine (L–H) ligand 1 afforded several new organometallic aluminium complexes. Reaction in a 1 : 1 stoichiometry at room temperature afforded a Lewis acid–base adduct, whereas thermolysis resulted in the loss of methane and the formation of a dimer complex, (L-AlMe2)2, 3. When reacted in a 1 : 2 ratio at 110 °C, the loss of two equivalents of methane yielded L2AlMe, 5, whereas when the reaction was performed at 60 °C, (L–H)AlMe2(L), 4, was found as an intermediate in the reaction. Compound 2 is, to the best of our knowledge, the first example of a structurally characterized primary imine coordinated to a triorganoaluminum(III) center. Attempts to form a two coordinate aluminum cation by CH3− abstraction are documented. All products were characterized via normal spectroscopic techniques and single crystal X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...