Introduction of taurine (2-aminoethanesulfonic acid) as a green bio-organic catalyst for the promotion of organic reactions under green conditions†
Abstract
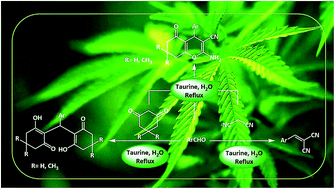
Taurine (2-aminoethanesulfonic acid), a semi-essential amino acid that exists in the human body and numerous other living creatures, is used as a green bio-organic catalyst for the promotion of the Knoevenagel reaction between aldehydes and malononitrile. In the same way, tetraketones can also be produced through a Knoevenagel reaction, followed by Michael addition. 2-Amino-3-cyano-4H-pyran derivatives are simply prepared via a three-component reaction in the presence of taurine as the catalyst. All these reactions are performed in water, a green solvent. The advantages of using of taurine as the catalyst are it is environmentally friendly, low cost, commercially available, easy to separate from the reaction mixture, and has high reusability. Use of this catalyst results in acceptable reaction times, high yields and high purities of the obtained products without utilizing any organic solvents.
- This article is part of the themed collection: Sustainable synthesis

 Please wait while we load your content...
Please wait while we load your content...