Synthesis of spiro isoindolinone-indolines and 1,2-disubstituted indoles from 2-iodobenzamide derivatives†
Abstract
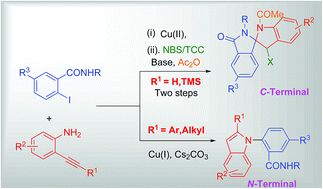
Copper catalyzed C-terminal and N-terminal attack of 2-alkynylanilines to 2-iodobenzamides afforded isoindolinones and 1,2-disubstituted indoles, respectively. Further, the isoindolinone derivatives were converted to spiro fused isoindolinone-indolines utilizing a halonium ion mediated strategy using NBS/TCC in the presence of acetic anhydride. Also, the ortho effect of the amide group in 2-iodobenzamide has been successfully applied for the synthesis of 1,2-disubstituted indoles.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives

 Please wait while we load your content...
Please wait while we load your content...