Ionothermal synthesis, magnetic transformation and hydration–dehydration properties of Co(ii)-based coordination polymers†
Abstract
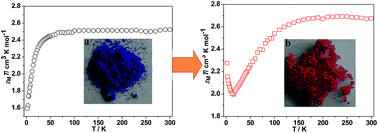
Two low-coordinated coordination polymers, [Co(nip)2][EMIm]2 (1) (H2nip = 5-nitryl-isophthalic acid, EMIm = 1-ethyl-3-methyl imidazolium) and [Co(bptc)][EMIm]2·H2O (2) (H4bptc = 2,2′,4,4′-biphenyltetracarboxylic acid) were prepared under an ionic liquid medium, demonstrating that ionothermal synthesis may act as a powerful tool in the preparation of low-coordinated coordination polymers (LCCPs). Investigation on the synthetic conditions shows that a lower reaction temperature, appropriate ratio of ligand to metal ions, and hydrophobic ionic liquid are of key importance for the formation of LCCPs. Both of them feature interesting hydration–dehydration properties with reversible color changing from deep purple to pink. Solid state UV-Vis spectra measurements also indicate that the largest adsorbtion peaks are blue-shifted under hydration. Significantly, under hydration and dehydration processes, compound 2 exhibits a magnetic transformation from anti-ferromagnetism to ferrimagnetism. Essentially, this transformation arises from the formation of new propagating ways triggered by guest water.

 Please wait while we load your content...
Please wait while we load your content...