Diastereoselective synthesis of novel tetra-and pentacyclic annulated coumarino-δ-sultam pyrrolidine, pyrrolizidine, pyrrolothiazole and isoxazolidine derivatives via intramolecular 1,3-dipolar cycloadditions†
Abstract
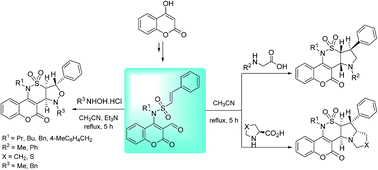
A facile and efficient strategy towards the novel tetra- and pentacyclic annulated coumarino-δ-sultam pyrrolidine, pyrrolizidine, pyrrolothiazole and isoxazolidine via intramolecular 1,3-dipolar cycloadditions of nitrones or azomethine ylides is described. These highly functionalized polycyclic scaffolds were obtained with high diastereoselectivity in high yields via condensation of the initially prepared coumarin-based bifunctional starting materials with sarcosine or N-phenylglycine, L-proline or L-4-thiazolidinecarboxylic acid, and methyl or benzylhydroxylamine. The proof of the structures relies on analytical investigation and X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...