Sigma-spacer regulated thiophenyl triazine conjugates: synthesis and crystal, electronic and luminescent properties†
Abstract
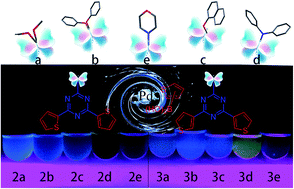
A water-accelerated Pd catalyzed Suzuki–Miyaura cross-coupling reaction was developed for the synthesis of thiophenyl triazine conjugates (TTCs). The compounds (2a–2e and 3a–3e) with five σ-spacers were obtained and fully characterized. The X-ray analysis of 2a, 2d, and 3d found that the length of the intermolecular H bond was 3.465(3) Å and the π–π interactions ranged from 3.6538(2)–3.9519(1) Å. Five σ-spacers of TTCs had an effect on their emissions from blue (363 nm) to yellow (530 nm) whilst 2- and 3-thiophenyl chromophores finely tune the emission from 530 to 504 nm. The DFT computation showed the TTCs had low HOMO (−6.8 eV) and LUMO (−2.5 eV) energy and high triplet energy (ET, 2.4 eV). The energy level and gap of TTCs were regularly modulated by the σ-spacer.

 Please wait while we load your content...
Please wait while we load your content...