Synthesis of diverse isatins via ring contraction of 3-diazoquinoline-2,4-diones†
Abstract
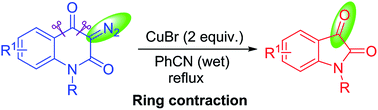
An efficient synthesis of diverse isatin derivatives was accomplished by a copper-mediated reaction of 3-diazoquinoline-2,4-diones via ring contraction through domino Wolff rearrangement, decarboxylation, bromination, substitution, and dehydration. This protocol has several advantages as a one-pot procedure, with functional group tolerance, and high yield.

 Please wait while we load your content...
Please wait while we load your content...