Pre-clinical risk assessment and therapeutic potential of antitumor lipopeptide ‘Iturin A’ in an in vivo and in vitro model
Abstract
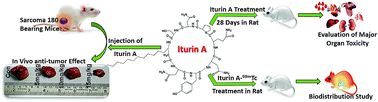
Lipopeptides are versatile bio-active weapons having antifungal, antibacterial, antimycoplasma and anticancer properties. In this study, the therapeutic potential and safety assessment of a lipopeptide molecule ‘Iturin A’ were evaluated. Iturin A was found to inhibit in vivo tumor growth in a sarcoma 180 mouse xenograft model. The antitumor efficacy of Iturin A was correlated with increased DNA fragmentation and modulation of CD-31, Ki-67, P-Akt, P-MAPK, apoptotic and anti-apoptotic proteins. Further, safety assessment was carried out in Sprague Dawley rats by 28 days repeated dose (28 days) toxicity and a bio-distribution study. In the toxicity study, Iturin A (10, 20 and 50 mg per kg per day) was administered to the animals for 28 days. Another group was kept for another 14 days without drug exposure after 28 days of treatment to access the reversibility of the toxicity. At the end of the treatment, body weight, food and water intake, organ weight, motility, hematology, serum biochemistry and histopathology of the major organs were evaluated. The bio-distribution of Iturin A was also performed in plasma as well as in different major organs by a well-developed and validated administration of Iturin A radiolabeled with 99mTc. The in vitro cytotoxic effect of Iturin A was also evaluated in BRL-3A rat liver cells. In the treated groups, various toxicities were found in the liver and spleen. However, these adverse effects were transient and reversible after discontinuation of Iturin A treatment. In conclusion, this pre-clinical study offered a preliminary investigation regarding the efficacy and safety assessment of Iturin A.


 Please wait while we load your content...
Please wait while we load your content...