A novel 3-D microporous magnesium-based metal–organic framework with open metal sites†
Abstract
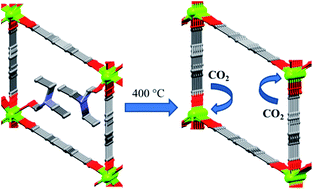
A novel 3-dimensional (3-D) Mg(II) metal–organic framework (MOF) [Mg4(bdc)4(DEF)4]n (1) was synthesized by the solvothermal reaction of 1,4-benzenedicarboxylic acid (H2bdc) and magnesium nitrate hexahydrate in N,N′-diethylformamide (DEF). Single-crystal structural analyses reveal that the bdc dianion connects two dinuclear units to form a tetranuclear unit. The dinuclear units consist of a Mg(II) ion that is tetra-coordinated to four bridging oxygen atoms and a Mg(II) ion that is hexa-coordinated to four bridging oxygen atoms, and two pendant DEF molecules. These special arrangements result in novel zig-zag patterned 1-D rhombic channels containing coordinated DEF molecules. Heating 1 to 400 °C provides a porous DEF-free MOF (3), as confirmed by thermogravimetric analysis (TGA), elemental analysis, powder X-ray diffraction (PXRD) and BET surface area. Remarkably, removing the coordinated DEF molecules from 1 while retaining the porosity might lead to the formation of open metal sites, which are favorable for the adsorption of various gas molecules. Adsorption experiments and ideal adsorbed solution theory (IAST) calculations show that 3 has much larger H2 and CO2 uptakes and a higher CO2/N2 selectivity than a sample activated at 300 °C (2), which still contains coordinated DEF molecules.

 Please wait while we load your content...
Please wait while we load your content...