Haloplumbate salts as reagents for the non-aqueous electrodeposition of lead†
Abstract
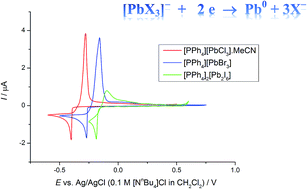
Cyclic voltammetry experiments on the Pb(II) salts, [PPh4][PbX3] (X = Cl, Br, I) in CH2Cl2 solution ([PPh4]X supporting electrolyte) at a Pt disk electrode show reproducible nucleation and stripping features consistent with reduction to elemental Pb. The reduction potential shifts to less cathodic from Cl (−0.40 V) → Br (−0.27 V) → I (−0.19 V vs. Ag/AgCl), in line with the Pb–X bond strengths decreasing. Potentiostatic electrodeposition using [PPh4][PbCl3] in CH2Cl2 leads to growth of a thin film of crystalline Pb onto planar TiN electrodes, confirmed by SEM, EDX and XRD analysis. Electrodeposition under similar conditions onto a planar Au electrode leads to deposition of elemental Pb, accompanied by some alloying at the substrate/film interface, with XRD analysis confirming the formation of AuPb2 and AuPb3. Transferring the [PPh4][PbCl3] reagent into supercritical CH2F2 (17.5 MPa and 360 K) containing [PPh4]Cl led to very limited solubility of the Pb reagent; using [NnBu4]Cl as a supporting electrolyte caused an increase in solubility, although still lower than in liquid CH2Cl2. Cyclic voltammetry experiments (Pt disk) using this electrolyte also show voltammetry consistent with Pb deposition, however the low solubility of the lead salt in scCH2F2 meant that electrodeposition onto a planar TiN substrate was not possible.

 Please wait while we load your content...
Please wait while we load your content...