Molecular modeling of structural and functional variance in the SAGA deubiquitinating module caused by Sgf73 Y57A mutation†
Abstract
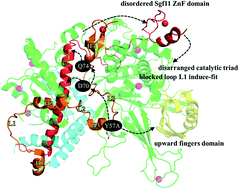
The Spt-Ada-Gcn5-acetyltransferase deubiquitinating module is composed of four protein subunits: Ubp8, Sgf11, Sus1, and Sgf73. Recent biomolecular data has suggested that a mutation of Sgf73 wherein tyrosine-57 (Y57) is replaced by a smaller alanine residue introduces structural instability to the entire module, leading to a loss of deubiquitinating function. Notably, although Sgf73 Y57 is not directly involved in ubiquitin substrate binding, it finely tunes the structure and dynamics near the active site through intraprotein communication pathways. Here, we assessed possible allosteric mechanisms caused by Sgf75 Y57A mutation through molecular modeling. The data obtained suggest that such a mutation has a direct impact on Ubp8's fingers subdomain, which harbors the ubiquitin substrate's globular portion, and has an indirect influence on Sgf11's N-terminal helix, whose instability gradually perturbs Sgf11's C-terminal ZnF domain and Ubp8's Cys-His-Asn catalytic triad.

 Please wait while we load your content...
Please wait while we load your content...