Base-driven keto–enol anion tautomerism of a perylene diimide derivative in DMF solution†
Abstract
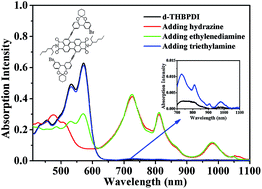
A perylene diimide derivative (d-THBPDI) containing oxygen bridged twisted heptatomic biphenyl substituents in the bay positions exhibited base (such as hydrazine, 1,2-ethylenediamine, triethylamine and K2CO3)-driven keto–enol anion tautomerism in DMF solution. The reduction of d-THBPDI (10−5 M, 2.5 mL) using hydrazine produced its radical anion and dianion with a hydrazine concentration of 6 μL and 60 μL, respectively. However, only the radical anion was obtained when 1,2-ethylenediamine, triethylamine or K2CO3 was added, even in large excess. Moreover, the reduction of d-THBPDI with different bases of the same amount was investigated, indicating the reduction of d-THBPDI was correlated to the basicity of the bases added. Furthermore, the base-driven tautomerism exhibited a reversibility with pH, which reflected on the transition between d-THBPDI and d-THBPDI˙− through adding bases and acid repeatedly. In addition, the reduction process of d-THBPDI also showed time and temperature-dependent features.

 Please wait while we load your content...
Please wait while we load your content...