Ru(ii)-p-cymene complexes containing esters of chiral d/l-phenylalanine derived aroylthiourea ligands for enantioselective reduction of pro-chiral ketones†
Abstract
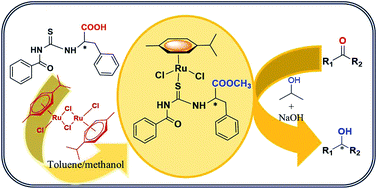
A series of new chiral aroylthiourea ligands was derived from unprotected D/L-phenylalanine: (R)/(S)-2-(3-benzoylthioureido)-3-phenylpropanoic acid (L1/L2), (R)/(S)-2-(3-(thiophene-2-carbonyl)thioureido)-3-phenylpropanoic acid (L3/L4) and (R)/(S)-2-(3-(furan-2-carbonyl)thioureido)-3-phenylpropanoic acid (L5/L6). Chiral Ru(II) complexes (1–6) were obtained from the reactions between the chiral ligands (L1–L6) and [RuCl2(p-cymene)2]2 through in situ catalytic esterification of the ligand in the presence of methanol solvent. The ligands and complexes were characterized by analytical and spectral (1H NMR, 13C NMR, Mass, FT-IR, electronic) techniques. The molecular structure of the ligand L1 showed the presence of an unprotected acid group and that of the representative complexes confirmed the conversion of acid to ester. The X-ray structure of two of the complexes (3 and 6) revealed the sulfur only monodentate coordination of the aroylthiourea ligands. All the chiral complexes turned out to be efficient catalysts for the enantioselective reduction of aromatic pro-chiral ketones in the presence of 2-propanol and NaOH to produce chiral alcohols in excellent conversions (up to 99%) and enantiomeric excesses (up to 99%) within 10–12 h.

 Please wait while we load your content...
Please wait while we load your content...