Thermally stable energetic salts based on 3-nitramino-4-tetrazolefurazan†
Abstract
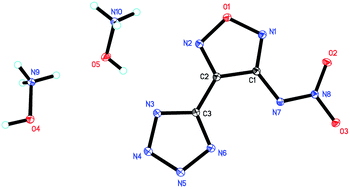
In order to develop new energetic materials with high energy and low sensitivity, a series of nitrogen-rich energetic salts based on 3-nitramino-4-tetrazolefurazan were conveniently synthesized through nitration, neutralization and metathesis reaction. All energetic salts were fully characterized by NMR (1H, 13C), IR and elemental analysis. Furthermore, ammonium salt (2) was confirmed by 15N NMR and hydroxylammonium salt (5) was confirmed by single-crystal X-ray diffraction. Salt 5 crystallized in triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with four hydroxylammonium cations and two NATF anions per unit cell at a density of 1.815 g cm−3. The densities of the salts are in the range of 1.621 (6) to 1.815 g cm−3 (5). Due to the two nitrogen-rich cations in the salts, they show high positive enthalpies of formation falling between 299.2 kJ mol−1 (2) and 1040.6 kJ mol−1 (9). All the salts show good thermal stabilities with the decomposition temperatures ranging from 203 °C (4) to 284 °C (6). The impact sensitivities of the energetic salts lie between 5 J (2 and 5) and >40 J (6, 7, 8) and their friction sensitivities range from 108 N (4) to >360 N (6). Their detonation performances were calculated according to Kamlet–Jacobs equations. The detonation pressures of the synthesized energetic salts were found to be in the range of 21.1 GPa (6) to 34.7 GPa (5) and their detonation velocities are between 7117 m s−1 (6) and 8826 m s−1 (5).
with four hydroxylammonium cations and two NATF anions per unit cell at a density of 1.815 g cm−3. The densities of the salts are in the range of 1.621 (6) to 1.815 g cm−3 (5). Due to the two nitrogen-rich cations in the salts, they show high positive enthalpies of formation falling between 299.2 kJ mol−1 (2) and 1040.6 kJ mol−1 (9). All the salts show good thermal stabilities with the decomposition temperatures ranging from 203 °C (4) to 284 °C (6). The impact sensitivities of the energetic salts lie between 5 J (2 and 5) and >40 J (6, 7, 8) and their friction sensitivities range from 108 N (4) to >360 N (6). Their detonation performances were calculated according to Kamlet–Jacobs equations. The detonation pressures of the synthesized energetic salts were found to be in the range of 21.1 GPa (6) to 34.7 GPa (5) and their detonation velocities are between 7117 m s−1 (6) and 8826 m s−1 (5).

 Please wait while we load your content...
Please wait while we load your content...