A 2-mercaptobenzothiazole-functionalized ionic liquid for selective extraction of Pd(ii) from a hydrochloric acid medium†
Abstract
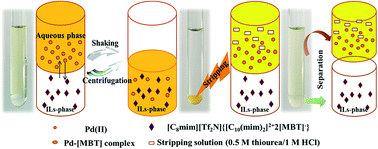
A novel 2-mercaptobenzothiazole-functionalized ionic liquid (1,10-bis(3-methylimidazolium-1-yl)decane bis(triflic)imide two 2-mercaptobenzothiazolate, {[C10(mim)2]2+2[MBT]−}) with excellent selectivity for Pd(II) extraction was fabricated. Variable factors (e.g., functional ionic liquid concentration, hydrochloric acid concentration, sodium chloride concentration, sodium nitrate concentration and hydrogen ion concentration) were also investigated for extraction experiments. Accordingly, a special complexation extraction mechanism was proposed and confirmed by further experiments. Moreover, higher selectivity towards Pd(II) extraction was realized over other metal ions especially Pt(IV). Furthermore, 0.5 M thiourea/1.0 M HCl was recognized as an efficient stripping agent for the Pd(II) loaded organic phase. Consequently, together with high efficiency and selectivity, the 2-mercaptobenzothiazolate-functionalized ionic liquid can act as a high selective extractant for Pd(II) extraction.

 Please wait while we load your content...
Please wait while we load your content...