Kinetic investigations of chlorine atom initiated photo oxidation reactions of 2,3-dimethyl-1,3-butadiene in the gas phase: an experimental and theoretical study†
Abstract
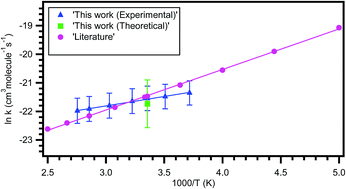
Rate coefficients for the reaction of chlorine atoms with 2,3-dimethyl-1,3-butadiene were measured over the temperature range of 269–393 K by using a relative rate experimental method with reference to isoprene and 1-pentene. Concentrations of the 2,3-dimethyl-1,3-butadiene and reference compounds were monitored by gas chromatography equipped with a Flame Ionization Detector (FID). The obtained rate coefficient for the reaction of 2,3-dimethyl-1,3-butadiene with Cl atoms at 298 K is k = (4.48 ± 0.46) × 10−10 cm3 per molecule per s, which is in good agreement with the reported rate coefficient at 298 K and 760 Torr. The obtained temperature dependent rate coefficient is k2,3-dimethyl-1,3-butadiene+Cl (269–363 K) = (4.59 ± 0.67) × 10−11 exp[(663.13 ± 45.8)/T] cm3 per molecule per s. To further understand the reaction mechanism of butadienes with Cl atoms, theoretical calculations were performed for the reactions of 2-methyl-1,3-butadiene (isoprene) and 2,3-dimethyl-1,3-butadiene with Cl atoms as a function of temperature using Conventional Transition State Theory (CTST) in combination with QCISD(T), CCSD(T) and MP2 levels of theory with different basis sets. The theoretically calculated temperature dependent rate coefficient is k2,3-dimethyl-1,3-butadiene+Cl (200–400 K) = (4.12 ± 0.38) × 10−12 exp[(1419.2 ± 26)/T] cm3 per molecule per s which is in excellent agreement with our experimentally measured rate coefficients. The rate coefficient was observed to be increasing with the substitution of hydrogen atoms by methyl groups.

 Please wait while we load your content...
Please wait while we load your content...