Two catalytic systems of l-proline/Cu(ii) catalyzed allylic oxidation of olefins with tert-butyl hydroperoxide
Abstract
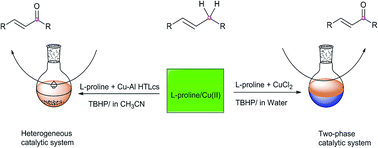
The nontoxic and water-soluble L-proline combined with two different forms of copper(II): recoverable Cu–Al hydrotalcite-like compounds (Cu–Al HTLcs) and water-soluble CuCl2, as a heterogeneous catalytic system (L-proline/Cu–Al HTLcs) and two-phase catalytic system (L-proline/CuCl2) to catalyze allylic oxidation with tert-butyl hydroperoxide. The results showed that L-proline/Cu(II) is highly active for oxidizing isophorone (IP) into ketoisophorone (KIP). Maximum catalytic effects were afforded respectively under the optimal reaction conditions, which obtained 77.9% IP conversion with 74.3% KIP selectivity catalyzed by L-proline/Cu–Al HTLcs and 73.5% conversion with 81.6% selectivity by L-proline/CuCl2. Recycling experiments of the two catalytic systems of L-proline/Cu(II) showed they are stable and recyclable for at least six cycles with appreciable catalytic activity. And various hydrocarbons could be smoothly transformed into corresponding ketones with satisfactory conversion and selectivity by the two catalytic systems.

 Please wait while we load your content...
Please wait while we load your content...