Covalent surface modification of α-MnO2 nanorods with l-valine amino acid by solvothermal strategy, preparation of PVA/α-MnO2-l-valine nanocomposite films and study of their morphology, thermal, mechanical, Pb(ii) and Cd(ii) adsorption properties
Abstract
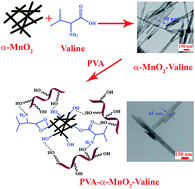
The surface of α-manganese dioxide (α-MnO2) nanorods was modified chemically with L-valine amino acid by a solvothermal strategy. The α-MnO2 nanorods were prepared by a hydrothermal method. Then poly(vinyl alcohol)/α-MnO2-L-valine nanocomposites (NCs) containing 1, 3 and 5 wt% of modified α-MnO2 nanorods were prepared through an ultrasound-assisted technique. Fourier transform infrared spectroscopy, X-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy and UV-visible spectroscopy were used to investigate and characterize nanostructures and NCs. Following this, the effects of α-MnO2-L-valine nanorods on the properties of NCs, such as the mechanical and thermal properties, were studied. The Brunauer–Emmett–Teller (BET) results showed that NC 3 wt% had higher surface area, pore volume and pore size than pure PVA with mesoporous structure. Finally, NC 3 wt% was investigated as an adsorbent for sorption of Pb(II) and Cd(II) ions. It showed good adsorption potential for the removal of Pb(II) and Cd(II) in aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...