Affinity adsorbents for proline-rich peptide sequences: a new role for WW domains†
Abstract
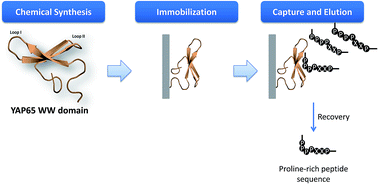
The WW domain derived from human Yes-associated protein (hYAP65_WW) recognizes proline-rich peptides. The structural and chemical robustness of WW domains makes them appealing candidates to target and capture these peptides in affinity purification processes. In this work, the chemical synthesis of the hYAP65_WW domain containing a terminal cysteine for oriented coupling onto the chromatographic matrix was successfully achieved by a fragment solution condensation reaction and by incorporation of pseudoproline dipeptide units. Both strategies yielded a hYAP65_WW protein with the characteristic WW domain folding. The purified hYAP65_WW domain was immobilized in a chromatographic matrix and tested for binding to a proline-rich peptide. The adsorbent bound 92 ng of peptide per mg of support and the elution was particularly efficient when employing a low pH or an increase in salt concentration. This work sets the ground for the application of WW domains as affinity reagents towards the capture and elution of peptides and proteins rich in proline sequences.


 Please wait while we load your content...
Please wait while we load your content...