Kinetics and thermodynamics studies for bisphenol S adsorption on reduced graphene oxide
Abstract
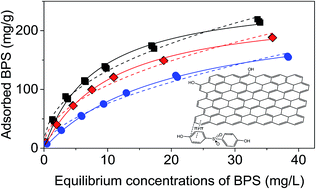
The adsorption behavior of bisphenol S (BPS) on reduced graphene oxide (rGO) was investigated. The adsorption kinetics data are best described with a pseudo-second-order model and the equilibrium data are well fitted by both Freundlich and Langmuir models. The maximum adsorption capacity of BPS on rGO at pH 6.0 and T = 298 K was 261.74 mg g−1. It was found that the increase of solution pH was unfavorable for BPS adsorption due to the increasing electrostatic repulsion between BPS and rGO. The thermodynamics data indicated that the adsorption of BPS on rGO was a spontaneous and exothermic process. Fourier transform infrared (FTIR) spectra suggested that BPS adsorption on rGO was primarily driven by π–π interaction between the graphene's hexagonal arrays and the aromatic core of BPS. Results from this study demonstrate that rGO is a promising adsorbent for BPS removal in water treatment.

 Please wait while we load your content...
Please wait while we load your content...